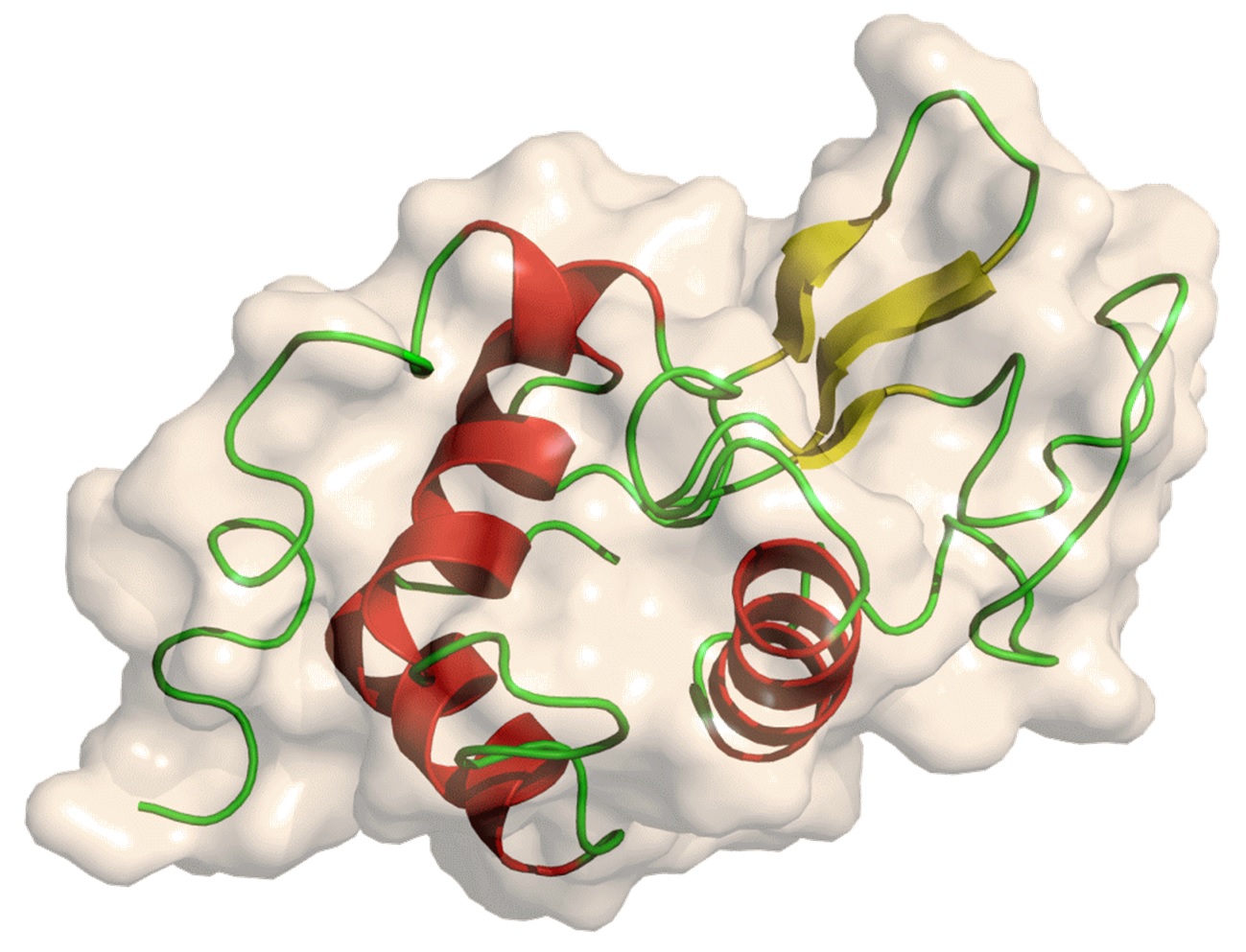
Introduction
Proteinase K is a powerful proteolytic enzyme isolated from Candida albicans, with high specific activity and is a key reagent for DNA extraction. This enzyme is active in a wide pH range (4-12.5) and at high temperatures (50-70 ° C), and is used for the isolation of plasmids or genomic DNA and RNA. In DNA extraction, the main function is to enzymatically hydrolyze histones that bind to nucleic acids, allowing DNA to free in solution. Subsequently, different methods are used for extraction to remove impurities and collect DNA. Neither chelating agents such as EDTA nor descaling agents such as SDS can inactivate it. The storage solution for Proteinase K is generally 10mg/ml or 20mg/ml. Store at -20°C. The Proteinase K solution is colorless and transparent. If precipitation occurs, it cannot be used again.
Proteinase K was purified from Candida albicans. According to the data, the enzyme has two Ca2+binding sites, which are at a certain distance from the enzyme’s active center and have no direct relationship with the catalytic mechanism. However, if Ca2+ is removed from the enzyme, due to remote structural changes, catalytic activity will be lost by about 80%, but its remaining activity is usually sufficient to degrade proteins that typically contaminate acidic products. Therefore, EDTA is usually added during the digestion process of Proteinase K to inhibit the action of nucleases dependent on Mg2+. However, if you want to digest proteins with strong resistance to Proteinase K, such as keratin, you may need to use a buffer containing 1mmol/l Ca2+ without EDTA. After digestion and before purifying the nucleic acid, EGTA (pH 8.0) should be added to a final concentration of 2mmol/l to chelate Ca2+.
Proteinase K is a serine protease with broad cleavage activity. It cleaves the carboxyl end peptide bonds of fatty and aromatic amino acids. This enzyme has been purified to remove RNA enzyme and DNA enzyme activities. Due to the stability of Proteinase K in urea and SDS, as well as its ability to degrade natural proteins, it is widely used, including preparing chromosomal DNA for pulse electrophoresis, protein blotting, and removing nucleases from DNA and RNA preparation. The general working concentration of Proteinase K is 50-100 μ G/ml. It is active over a wide pH range (pH 4-12.5).
Storage buffer
Recommended reaction buffer: 50mM Tris HCl (pH 7.5), 10mM CaCl2.
Preparation standard for 20mg/ml Proteinase K: Add 200mg of Proteinase K to 9.5ml of water, gently shake until Proteinase K is completely dissolved. Do not vortex mix. Add water to a constant volume of 10ml, then divide into small portions and store at -20℃.
Inhibitor
Proteinase K is a serine protease, therefore its action can be inhibited by benzylsulfonyl fluoride, which is an inhibitor of serine proteases.
Application
- DNA and RNA extraction
Proteinase K can degrade proteins that bind to nucleic acids, promote the separation of nucleic acids, and has great advantages in extracting high molecular weight nucleic acids. Proteinase K is also commonly used for the inhibition and degradation of RNase during RNA extraction. The quality of RNA is crucial for the success or failure of Northem blotting and hybridization analysis, cDNA synthesis, and in vitro translation experiments. However, RNA is extremely unstable and easily degraded by widely existing RNases. Therefore, creating a non RNase environment using proteases and strictly preventing RNase contamination are the key to successful RNA extraction. - in situ hybridization
Proteinase K is commonly used in in situ hybridization technology for sample processing before hybridization. It has the function of digesting and surrounding target DNA proteins to facilitate probe penetration and improve detection sensitivity. - Application of inactivating bacteria in domestic and industrial water
The outer structure of viruses is generally dominated by capsid proteins, and Proteinase K can disrupt the binding of viruses to host cells by disrupting the capsid proteins. Under certain conditions, the virus inactivation rates in pure water and domestic sewage were 99.4% and 49.4% respectively after treatment with Proteinase K at a concentration of 67.51ug/mL for 1 hour. The inactivation rates after treatment for 3 hours were>99.9% and 81.8%, respectively, indicating a significant effect on virus inactivation. The advantage of using Proteinase K for virus inactivation is that the disinfectant itself is harmless and does not produce harmful disinfection products, so this is an efficient and environmentally friendly method. - Meat tenderization
Proteinase K can break down muscle fibers, connective tissue proteins, and even elastin and collagen in meat, causing changes in the spatial structure of various levels of protein, partial peptide bond breakage, water absorption and swelling of meat, and tender texture, meeting consumer requirements for meat quality. - Biological tests such as COVID-19
For COVID-19, the treatment, extraction and amplification during the nucleic acid amplification test are high-risk, so it is necessary to inactivate the virus in the sample before amplification. Proteinase K is an important component of the virus sampling solution, which can lyse the virus to release nucleic acid and eliminate the nucleic acid lyase to prevent the degradation of virus RNA. And it can inactivate the virus, denature its protein, lose its activity and “die”, no longer have infectivity, and improve the safety of transportation and testing stages.
Difficulties in the production of Proteinase K
- Low expression level
Proteinase K can degrade most proteins non-specific and cause severe toxicity to host cells, so the expression level of Proteinase K is generally low. Screening expression systems and strains with high expression of Proteinase K generally requires a longer cycle. - Residual pigments and nucleic acids
Large scale fermentation introduces a large amount of residual pigments and host nucleic acids, making it difficult to remove these impurities with simple purification processes. However, complex purification increases costs and reduces recovery rates. - Instability
Proteinase K is not stable enough and can hydrolyze itself. Without a protective agent, it is difficult to store it stably for a long time at 37 ℃. - Easy to precipitate
When preparing freeze-dried powder of Proteinase K, in order to ensure a high solid content of Proteinase K in the freeze-dried powder, it is necessary to add freeze-dried protectants at high concentrations. However, when the concentration of Proteinase K reaches 20mg/mL or above, it is easy to aggregate and form precipitates, which brings great difficulties to the high solid content freeze-drying of Proteinase K. - Large investment
Proteinase K has strong protease activity and can hydrolyze other proteases in the laboratory, so it requires specialized production areas, equipment, and personnel for research and development.
Our company’s Proteinase K comes from Pichia pastoris, which expresses the Proteinase K gene of Engyodonium Album, which has been optimized through site directed mutagenesis. It has advantages such as high specific activity, high yield, and a wider range of temperature/pH value activity. Advanced protein engineering technology and a 28000 liter yeast platform effectively reduce unit costs, providing the most cost-effective Proteinase K for biopharmaceutical research and production. The efficient expression of yeast and chromatographic purification ensure the purity of the product. This product does not contain bacterial endotoxins and has a wider range of applications than natural Proteinase K.
| Appearance | White or almost white freeze-dried powder |
| Enzyme activity | ≥ 30U/mg freeze-dried powder |
| Optimal pH | 7.5-11.5 |
| Optimal temperature | The recommended temperature range is 37-70℃, with the highest activity at 70℃ |
| Inactivation method | Adding 5mM of PMSF to the reaction system can lead to deactivation |
| Unit definition | The amount of Proteinase K that can hydrolyze casein substrate to produce 1µmol tyrosine per minute at 37℃ and pH 7.5 is defined as one unit (U). |
| Impurity residue | No DNA and RNA, no DNase and RNase. No deoxyribonuclease activity was detected when digested with λDNA as a substrate at 37℃ for more than 3 hours. No ribonuclease activity was detected when RNA was used as a substrate and digested at 37℃ for more than 3 hours. |
| Electrophoretic purity | ≥ 95% (Native Page) |
| Activator | 1-5mM Ca2+ |