Bst 2.0 Pro DNA Polymerase
2024-07-03
TRANS1000 transfection reagent
2024-10-18T7 RNA Polymerase, GMP Grade
Product Number: GMP-T701 Animal-free Ampicillin-free
Shipping and Storage
At -20±5℃.
Description
As a biological macromolecule, mRNA can be synthesized on a large scale by in vitro transcription (IVT). T7 promoter is one of the most efficient promoters at present. Therefore, T7 RNA polymerase can be used for in vitro transcription to obtain more synthetic products. T7 RNA polymerase is a T7 promoter-specific, DNA-dependent, 5’→3′ RNA polymerase from T7 bacteriophage. Usingdouble stranded DNA as the template, it transcribes RNA complementary to the single stranded DNA located at the downstream of T7promoter. T7 RNA polymerase has been commonly used for in vitro mRNA synthesis.
The polymerase is GMP Grade produced in E. coli. Our manufacturing processes are strictly controlled to ensure the end products free from host protein or nucleic acid contaminations and other impurities following the Pharmaceutical Manufacturing Guidelines. We guarantee the manufacturing and quality control comply with GMP regulation for tracking each and every step of the manufacturing process, including raw material sourcing.
This product has completed the DMF record of FDA and passed the HALAL certification.
Quality Elements
| Element | Standard |
| Appearance | transparent liquid |
| Visible impurities | complying to regulation |
| pH value | 7.5-8.5 |
| Active | 49kU/ml-51kU/ml |
| purity | ≥95% |
| Endonuclease residues | The degradation of substrate was ≤10% |
| Exonuclease residues | The degradation of substrate was ≤10% |
| RNase residues | The degradation of substrate was ≤10% |
| Endotoxin residues | <5EU/mg |
| Exogenous DNA residues | ≤100 pg/mg |
| Host protein residues | ≤50 ppm |
| Mycoplasma | Negative |
| Heavy metal residues | ≤10 ppm |
Annotation: ChP refers to the Pharmacopoeia of the People’s Republic of China.
Complying to following regulations
- ISO 9001:2015, certified facility.
- 《GMP Appendix – Cellular therapeutic product》National Medical Products Administration.
- 《The Pandect of Genetic Therapeutic Product for Human》Chinese Pharmacopoeia Commission.
- USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products.
- USP Chapter <92>, Growth Factors and Cytokines Used in Cell Therapy Manufacturing.
- Ph. Eur. General Chapter 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
Feature
Highly specific for T7 promoter, suitable for RNA in vitro synthesis.
Application
- Single stranded RNA synthesis
- RNA probe synthesis.
- siRNA precursor synthesis
- Precursor for RNA splicing preparation
- Capped RNA synthesis.
Examples
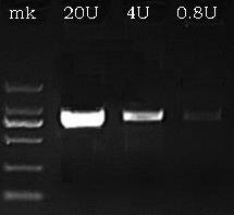
Fig: RNA transcription in vitro.
- From left to right, the quantity of T7 RNA Polymerase copies were 20U, 4U, 0.8U.
- DNA templates were segments of 2Kb.
Unit definition
At 37℃, pH8.0, within 1 hour, the amount of enzyme required that will incorporate 1nmol tritium labeled GMP into acid-insoluble material is defined as one unit of enzyme activity.
Storage buffer
100mM NaCl; 50mM Tris-HCl (pH 7.9); 1mM EDTA; 20mM 2-mercaptoethanol; 0.1% Triton X-100; 50% (v/v) Glycerol。
Related Products
TR02 – Thermostable T7 RNA Polymerase
TR03 – T7 RNA Polymerase 200U/ul